Underrated Ideas Of Tips About How To Build An Electrolytic Cell

The electrolytic cell is primarily utilized for the production of hydrogen gas and oxygen gas.
How to build an electrolytic cell. There are two situations that factor into the. In other words, a chemical reaction is used to produce electrical work (like powering a light bulb). Make sure you thoroughly understand the following essential ideas.
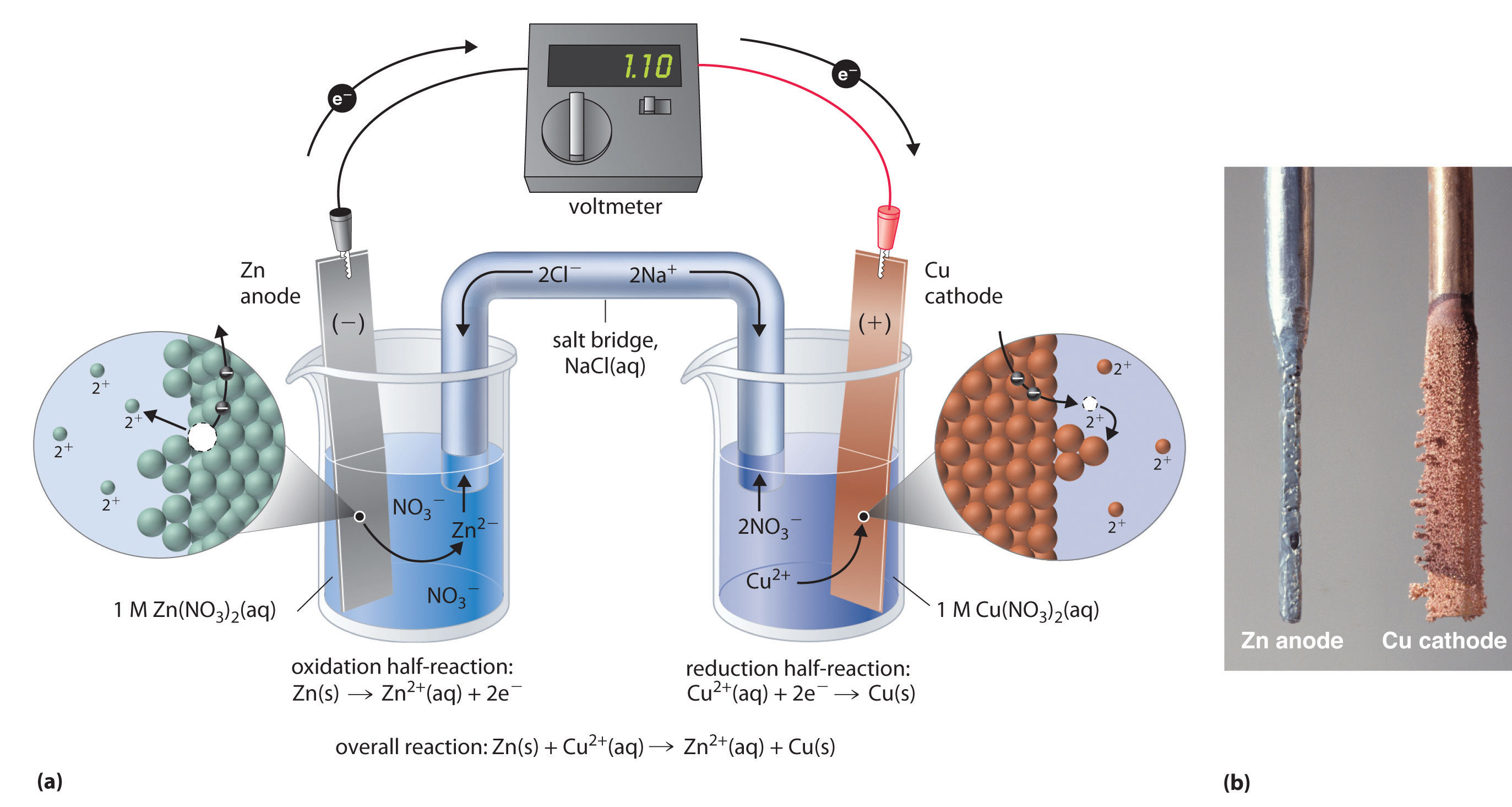
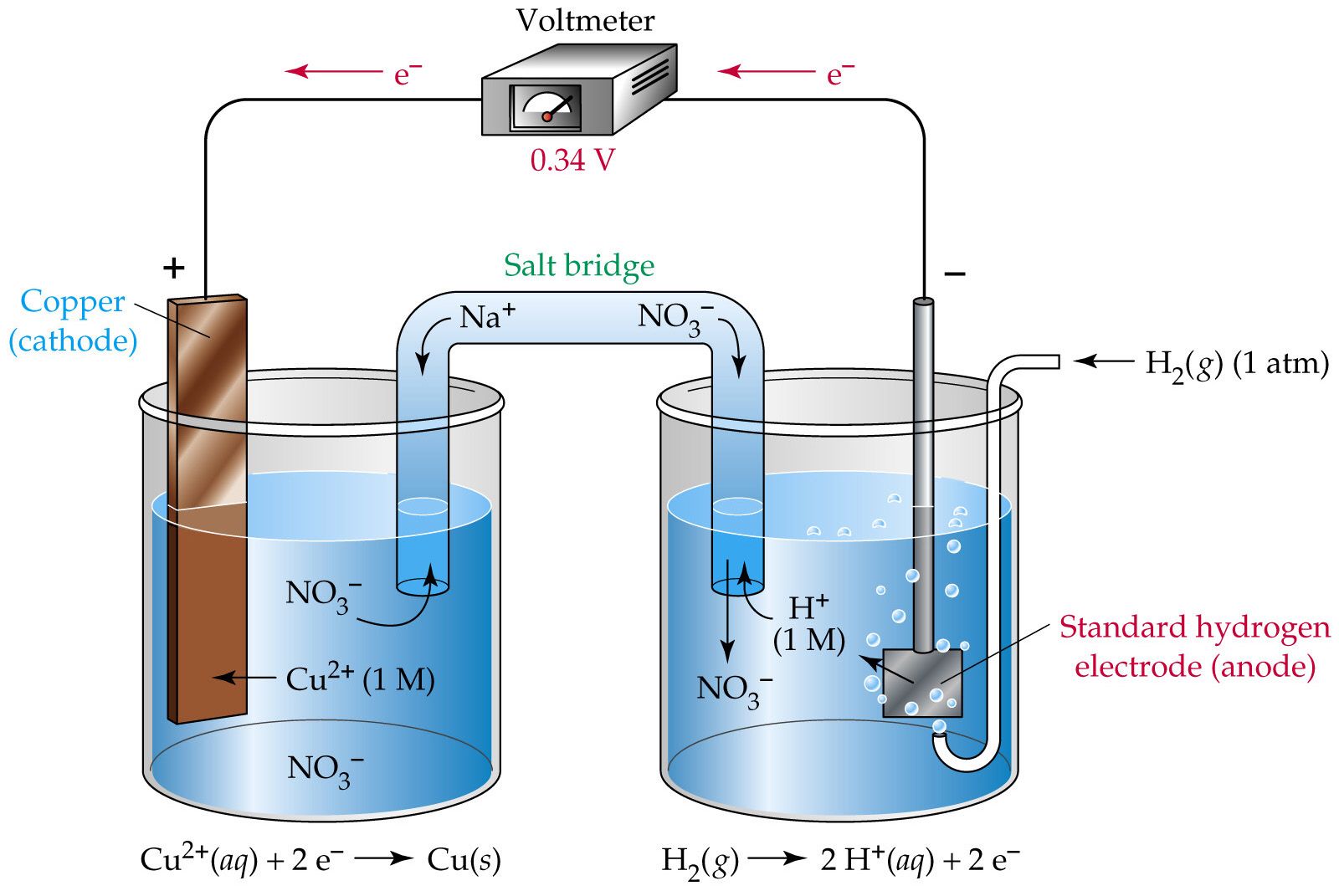
Have you ever wondered how a battery works? An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is. In a galvanic cell, the.
Electrolysis is responsible for the. Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy.
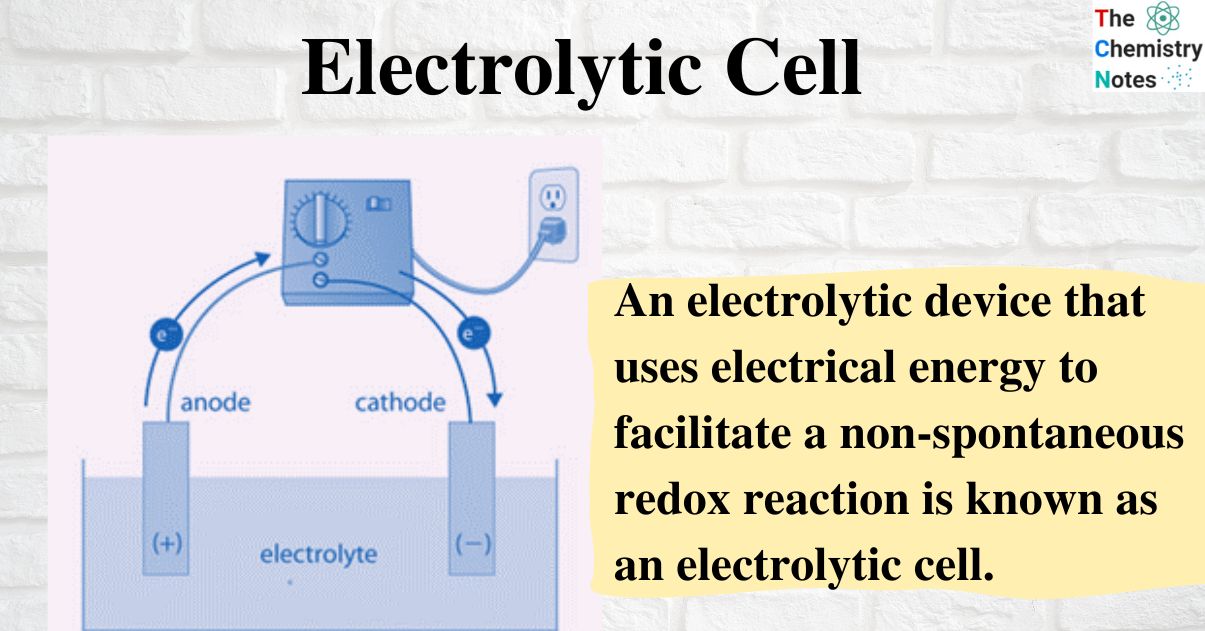
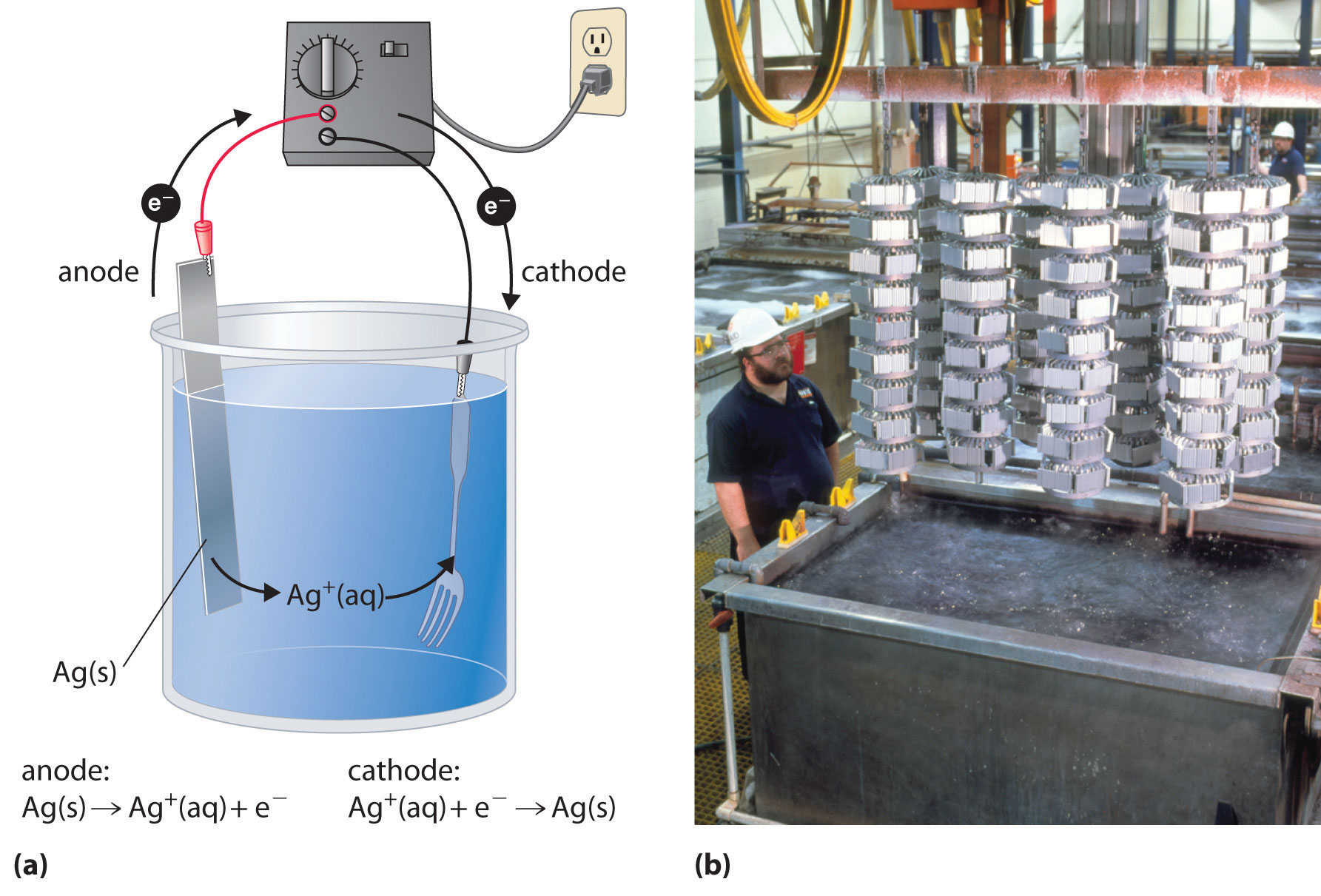
In an electrolytic cell, the redox reaction is thermodynamically unfavorable. I ramble a little at the beginning about electrolysis in. How to make an electrolytic cell what you need:
Demystify cells and batteries, and help students learn the underlying chemistry of galvanic cells with this simple microscale. However, the opposite is also possible. We explore the utility of electrolysis and build an electrolytic cell.
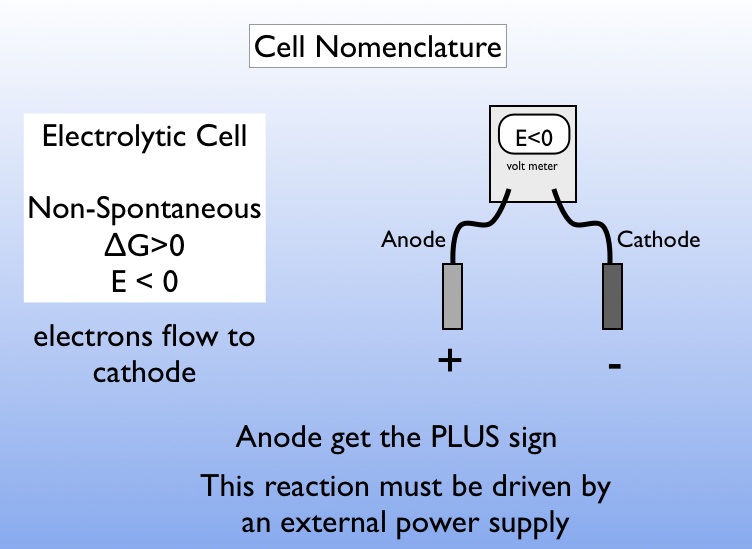
The electrolytic cell (unlike what we discussed above) is not driven by a spontaneous reaction and therefore requires an external. Goldman sachs estimates the global hydrogen market’s value may double to $250 billion by 2030, driven by the arrival of green hydrogen. Because the redox reaction is.
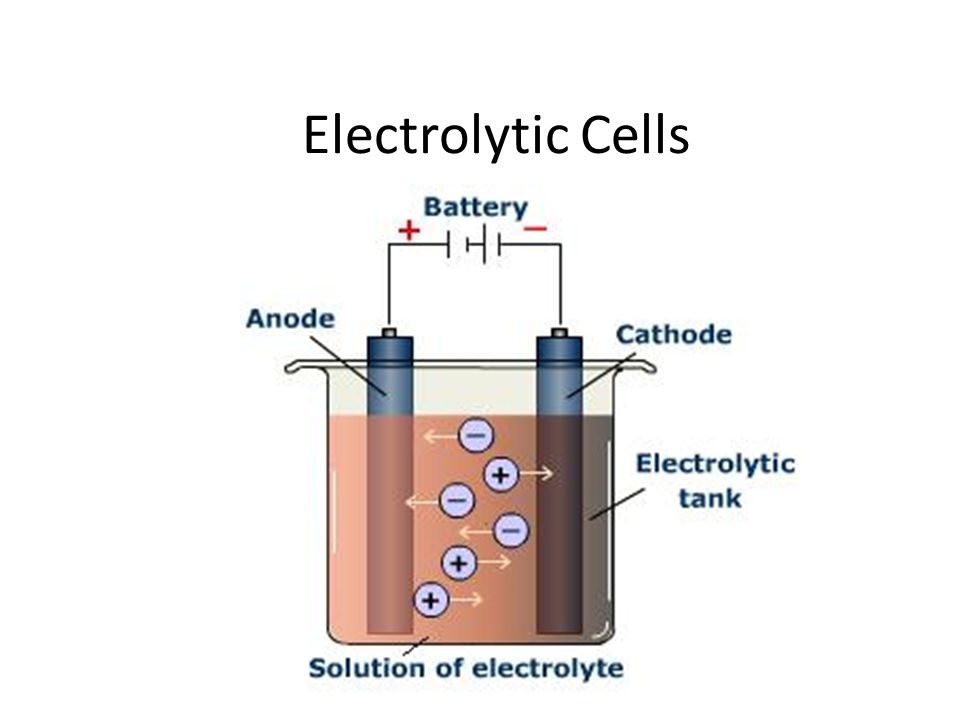
Many chemicals like caustic soda, chlorine gas, etc., are produced on a. It is possible to construct a cell that does work on a chemical system by driving an electric current through the system. In electrolysis, an external power source supplies the free energy required to drive a cell.
Smaa koraym at johns hopkins. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: This is part 1 of a short series exploring electrolysis.
Let's look at a quick summary for an electrolytic cell. These cells are called electrolytic cells. A redox reaction is split up into half cells, and you have an oxidation at the anode produc.
Protocol to watch the full video start a free trial today procedure electrolytic cells results procedure expand print procedure steps source: